
|
|

|
RSS-feed directory: |
Nikolay Timofeev, Research-Production Enterprise "CF BIO"; Russia |
Leuzea in VKontakte.Ru
|
| ORDER - Leuzea-powder, Serratula-powder, Seeds FORUM - "Leuzea, Ecdysterone and Serratula" |
|
|
Note:The paper is published (with updatings): |
|
In this paper, the actual level of scientific investigations on ecdysteroids is reviewed representing a promising and rapidly developing area of biomedical chemistry: fields of application, medical importance, major representatives, sources, and biological activity. Historical retrospection views and the achieved re-search levels are shown while the world flora screening, identification of most active formulas, and study of practical application possibilities. The Internet-resources (data bases, volume, availability) at electronic li-braries, search servers are analyzed for fundamental investigations and applied now-how, commercial offers.
Basic sources and biotechnological methods of production of the major representatives of ecdysteroids - ponasterone, muristerone, and ecdysterone are surveyed. It is emphasized that chemically isolated ecdys-teroids are extremely expensive and asked for mainly in science intensive investigations. To satisfy the mass demand for ecdysteroids in the pharmaceutical industry, non-purified or weakly purified plant formulations from super producer species with null toxicity rate that do not require high-expensive processing technolo-gies have good prospects.
In the case of Russia, cultivating Rhaponticum and Serratula plants representing super concentrator plant species is economically sound. The basic ecdysterone concentrations in plants of Rhaponticum carthamoides (Wlld.) Iljin - Leuzea or Maral root and Serratula coronata L. comprise 0.12-0.57 % and 0.31-1.15 % dry weight, respec-tively. Regarding the former species, there has appeared an industrial growing technology and a new class of pharmaceutical preparations from its aboveground shoots is being developed. The effective biological activ-ity rate of extracts from Rhaponticum carthamoides grown with a special technology in agropopulations accounts for 10-11...10-13 M, that is 3-4 orders of magnitude higher than the activity rate of highly purified individual ecdysteroids (0.5-10 µg/kg against 5-50 mg/kg).
Keywords: ecdysteroids, 20-hydroxyecdysone, ponasterone, muristerone, Rhaponticum carthamoides, Bioinfusin
One of the most important achievements of science in the last years is providing technologies on the plant-synthesized ecdysteroids? usage to control the growth and development parameters of different life or-ganisms. In addition to well-known adaptogenic and immuno-modulating properties of ecdysteroid-containing preparations used in classical and folk medicine (http://insectsciense.org/3.7; http://www.sciteclibrary.com/rus/catalog/pages/1502.html), this last discovery becomes even more signifi-cant and urgent for peoples health. Presenting ligands for intracell and membrane receptors, their regulating elements, ecdysteroids have an ability to change the homeostasis processes in organism, influencing cell growth, differentiation, and programmed death [37], production of specific products of metabolism. The role of ecdysteroids as ligands is switching between two states of gene transcription mechanism according to the switch on/switch off principle, and/or membrane transmission of signals to intracell targets via a series of secondary messengers.
In practical medicine, ecdysteroid-containing formulations are applied to prevent illness and preserve the immune status of healthy people [39, 53, 98]; they take an important part in sport, cosmic, and military medicine as adapting and work efficiency increasing drugs under limiting factors, e.g. when overcoming extreme physical and psychical efforts [82, 88, 89]. They are usable in transplantations of human organs and skin [59], to increase the hair growth, cure wounds, ulcers, and burns [43, 78]; they improve sexual function, stimulate li-bido and obviate difficulties in sexual life [34].
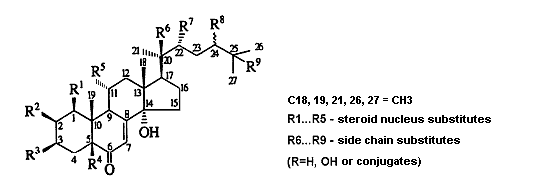
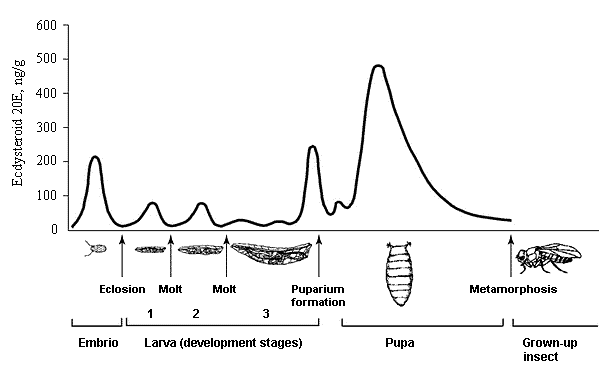
The ecdysteroid molecules (Fig. 1), presenting a group of lipophilic poly-hydroxylated steroids, par-ticipate in the life activity sustainability of practically all classes of organisms, while fulfilling numerous functions. The question on their role in living nature is still open. Certain is only the fact that one of the major ecdysteroid representatives, 20-hydroxyecdysone (20E), is a true molting hormone of arthropoda (insects and crustaceans) and initiates changes that occur at different developing stages from larva to chrysalis and then to a grown-up insect [36, 69].
Since appeared several thousand million years ago, ecdysteroids have participated in a complex co-evolution pathway of ecosystems development and adaptation to the environment. The presence of ecdyster-oids is characteristic, together with flowering plants, of such ancient organisms as ferns, mushrooms, mosses, algae, gymnosperms (Fig. 3). It is accepted that insects, which appeared at the later evolution stages com-pared to plants, started using them as hormonal factor of evolution. Because ecdysteroids as hormones show effects in extremely low concentrations (10-8...10-9 M), it is proposed that their increased synthesis by an-cient ferns and gymnosperms initially protected plants against phytophagous insects.
 |
|
Fig. 1. The structural formula of ecdysteroids |
 |
|
Fig 2. Ecdysteroid titer in the Drozophila melanogaster development (according to [36] changed) |
In the 60-ies years of XX century, the discovery of colossal amounts of molting hormones in plants (mil-lion times higher than in insects) caused a great scientific sensation. This discovery was proposed to contribute to an ecologically safe and quite effective method for the insect population control. Nevertheless, as the more detailed research has shown, most of insects are resistant to phytoecdysteroids (http://www.sciteclibrary.ru/rus/catalog/pages/4723.html) or learnt how to detoxify the hormones [14, 71] penetrating into their organisms through the mouth and started synthesis of their own zooecdysteroids (ecdysones) using other metabolic pathways differing from those of plants.
However, a 20-year-old investigation work in a field of cell and
molecular biology, ecological genetics and physiological sciences has led to
even more significant discoveries:
- ecdysteroids represent natural and
absolutely safe ligands in molecular systems of gene switching [10, 30, 56,
67];
- mechanisms on ecdysone- (ecdysteroid-) induced gene
expression systems like those existing in insect cells are applicable for
mammals, the human including [2, 15, 51];
- the above systems can be in vitro constructed, modified, and cloned by getting recombinant receptor proteins and transcription activators
on the basis of steroidal, tyroidal, retinoidal insect and mammal receptors, retro- and alfa-viruses, bacteriophages, and shock proteins [1, 31, 45, 60, 62].
The importance of the last discoveries becomes greater in the post-genome medical era, and large sums are expected to be invested in the development of molecular and gene therapy [32]. Upon completion of the human genome library sequencing, it is proposed that switch genes will allow switching off cells which produce organism-destroying structures (e.g. cancerous growth) and bringing to a stop diseases, incurable by conventional treatmen' modes (many inherited diseases) [37, 68]. Analogically, it will be possible to implant and pointwise switch on genes not present in the host cells but responsible for producing target therapeutic agents, as well as to set off the regeneration factors of damaged tissues [48].
Ecdysone-induced systems would also be applicable in research of group gene functions in both in vivo and in vitro systems. In the case of integration of such systems with modern computer technologies, there appears a possibility of diagnosing properties of every substance or biological object taken in a very small quantity (within 1 ng) [4]. For example, checking such systems with a laser ray for flourescent radiation occurring due to the coupled gene group expression in response to a medicine induced, and then com-paring it to the known profile, we can predetermine its mutageneity and citotoxicity rates [18].
Though the new directions on ecdysteroid application seem to be quite unusual, ecdysone-induced sys-tems are not only created and patented but also realized for commercial purposes (http://www.invitrogen.com; Fig. 4). Moreover, important aspects on clinical application of ecdysteroids are their participation in numerous non-genome effects. Despite the mechanisms on interconnection of ecdysteroids with membrane receptors as signal molecules, which activate secondary messengers, have been just recently put under investigation [11, 68], ecdysteroid-containing preparations are broadly used in practical medicine when curing cardio-vascular, nervous, and reproduction system diseases, whatever disorders of the whole homeostasis processes [16, 98].
That is why, for today we need those sources of ecdysteroid molecules or ecdysteroid-related com-pounds that would act in small quantities, be highly active, not toxic, resistant to decomposing influence, quickly removable from the organism, cost little, and could be produced in large dimensions [39, 50, 51].
Note. The consumer market of ecdysteroid-containing compounds does not stay within medicine but also in-volves the market segments concerning:
|
|
|
Fig. 4. The ecdysone-induced gene expression system based on the |
First investigations on ecdysones aiming isolation of insect hormones began in the early 30s and were conducted by the German scientists. In 1954, they managed to isolate 25 mg of weakly purified substance from 500 kg of silkworm chrysalides (Bombix mori) and to crystallize it. In 1963, as its general structure was discovered, α-ecdysone was related to steroids (with molecular weight M=464). In 1965, the molecular structure of α-ecdysone was determined by the X-ray structure analysis, which proved the absence of OH-group at carbon atom C20, compared to β-ecdysone (Fig. 5). These studies themselves were familiar only to a limited number of specialists and, possibly, the things would not have changed for a long time, if it were not for the concurrence of circumstances elicited a great interest and big investments in the investigations deal-ing with the world flora screening and research of new molecules properties.
The discovery of ecdysones in plants happened by a lucky chance when the scientist Karel Slama (Czecho-Slovakia) went to the USA to cultivate there a soil insect Pyrrhocoris apterus L. on filter paper. Here he was surprised - the insect metamorphosis was disturbed, and he could not obtain puration in the last larval stage. The key to explaining this phenomenon lay in filter paper: in that case it was produced from balsam fir (Abies balsamea). Other papers did not influence the metamorphism. The extraction procedure allowed for isolation of juvabione, structurally related to a juvenile hormone, which has selective effects on this single insect. Testing any other plants revealed that they contain numerous compounds with insect hormone activity. The biotest method was later modified in BII-biotest and to date is broadly used for the pri-mary screening of vegetal ecdysteroids, together with the radioimmune analysis (RIA).
Taking into account the economical and biological importance of phytoecdysones, the last three dec-ades were characterized by making significant efforts by screening the world flora to discover species pro-ducing most ecdysones, identify most active formulations, and to study possibilities on practical usage of ecdysones in different biology and medicine fields.
Ponasterone was a first phytoecdysterone isolated and described in 1966 by the Japanese scientists from the conifer Podocarpus nakaii [44]. Afterwards, it was identified in the affined species Podocarpus macrophyllus and Podocarpus reichei with concentration 100 g/kg dry wt [28, 57], and in the yew-trees Taxus canadasis, T. chinensis, T. cuspidata (50-80 mg/kg). In the late 70s and early 80s, ponaster'ne was detected in cancroids [38, 42], while in 1995 in flat-cap mushrooms (Paxillaceae) with concentration up to 50 mg/kg [63].
Ecdysterone (α-ecdysone, 20-hydroxyecdysone, 20E) was first isolated in 1966 from the crustacean Jasus calandei with a quantity of 2 mg/t and, therefore, named crustedysone [21]. Then, it was found in insects, silkworms Bombix mori and Antharea pernyi, and extracted with 200 mg 31 kg chrysalides [25]. In the same year the structure was assigned, and ecdysterone was isolated from conifers and ferns: first 50 mg/kg of Podocarpus elatus [26], further 10 g/kg Polipodium vulgare rhizomes [24].
Lately, ecdysterone was found in most plants [12], including cereals (maize - Zea mays) and crucifers (Arabidopsis thaliana). The concentrations differ by 1 million to 1 milliard times (20-300 ng to 20-30 g/kg). The general native sources on ecdysteroid industrial production are the perennial plants Rhaponticum carthamoides (Willd.) Iljin and Serratula coronata L., induced in different regions of Russia.
Muristerone A, the most active, rare and extremely expensive ecdysteroid for today, was found in 1972 by the German scientists in endemic plants seeds, genus Ipomoea [7], which occupy southern slopes of the Himalayas. The empomoea presents the most mysterious ecdysteroid source. Since the discovery of muristerone A, more than 1.5 thousand articles have been published on various aspects of its research but only some very first papers mention the source [7, 8, 9]. It appeared because the empomoea nomenclature is highly looped: it can mean completely diverse plants, often endemic [3]. Only 30 years later, there occurred new messages on muristerone isolation from sequoia-trees, not scientifically proven yet (http://www.sequoiasciences.com).
|
|
|
Fig. 5. Representatives of ecdysteroids |
Foreign resources. By the key word ecdysteroids, the scientific search server Scirus lists more than 2.5 thousand articles classifying them into 15 sections. The results about scientific research for the last 35 years are systematized and presented in a form of abstracts on such big servers as emngenta (ingenta.com), NCBI (ncbi.nlm.nih.gov), Synergy (blackwell-synergy.com), PubMed, Medline, BioMedNet (bmn.com). The server BioMedNet can be entered via the Russian branch BioMail (molbiol.edu.ru/cgi-bin/biomail), the Med-line via the Russian Found for Basic Research (RFBR) (intra.ru). Access to the sources is provided upon im-plementation of a few procedures, restriction concerns full text versions, only (for pay).
The Russian authors in foreign libraries are represented by single publications. Full versions of many articles are present on the PubMedCentral (pub.med.central.nih.gov), free admission to journal articles is al-lowed on the High Wire. There is a special site Ecdyzone (quasimodo.versailles.inra.fr/ecdyzone), devoted to the insect ecdysteroids. Access to patent sources is provided via the US Patent & Trademark Office. Infor-mation on structures, characteristics, properties of all known and newly discovered ecdysteroids is located on the server EcdyBase: http://ecdybase.org.
Russian resources. After entering the key words, the search systems Yandex, Aport, Rambler show from several to hundred sources. Recently, there has appeared a trend for enlarging a number of net sources the systems use. From the Russian electronic library catalogues, quite enough of the material is accumulated on the server Central Scientific Agricultural Library (www.cnsxb.ru) in the sections of general biology, biochemistry, plant physiology, biotechnology, botany, veterinary medicine.
Till recently, the most convenient, B, quickly-processed database (patent database including) was located on the Integrum-Techno (integrum.com). The defect of this server, the same as of other Russian resources, lies in absence of short material description and access commercialization. In the seek mode, there are only names of publications, which is explicitly insufficient for evaluating the importance and significance grade of the material. On the special request provided that it is permitted by the administration, a trial access to a limited amount of information resources (under 1 Mb) is provided for free.
On the RFBR (Russian Fund for Basic Research; elibrary.ru), the subject is poorly discussed, the elec-tronic library does not almost contain references to the Russian sources, access to the full text versions is not permitted. On the server CSML (Central Scientific Medical Library; scsml.rssi.ru) we failed to find any ecdysteroid sources. The database on ecdysteroid-containing plants growing in the northeastern part of Euro-pean Russia is collected on the server of the Institute of Biology Komi SC UrD RAS, where nearly one third of the regional species diversity is described (http://ib.komisc.ru/biochem/ecdysteroids).
Thus, the main defects of the Russian Internet resources are low request processing rate, absence of short material description, and total commercialization of access to full articles, if any. A characteristic fea-ture of the foreign resources is a global net unification of major organizations. Their servers are notable for high information proceeding rate, software and hardware service optimization. The request processing rate is much higher (sometimes by tens of times) than that of the Russian sources. The defects arise from the false presentation form of graphics and tables: it is not quite appropriate for slowly-working communication lines. The quickest databases are represented exclusively by texts, while graphics and tables are loaded in the alternative mode.
By the key words, the metasearch system AltaVista displays to one half-thousend references being the larg-est-scale information-processing system. Most of all, these are data about web pages of single authors and organi-zations. The on-line market is largely represented by highly purified ecdysteroids (Table 1) isolated from plants (the Aldrich-Sigma, Biochemicals Net, Invitrogen, Latoxan corporations, Russian Northern Biochemical Company etc.). The commercial firms (the Cytodyne Technologies, Gero Vita, Life Science Technologies, Natural Elixir; the Russian firm Mirra etc.) hold a special position in this series offering a number of ecdysteroid-containing preparations from natural material.
There are over tens of companies offering biologically active food additives from powder (tablet) ecdysterone against stress and psychical tiredness, to increase powers of physical endurance, develop muscles and improve the body (bodybuilding, fitness sports). Curiously enough is that till recently most cloned bio-preparations differing only by insignificant changes in their formulas were mainly composed of 20-hydroxyecdysone, isolated from the Rhaponticum carthamoides (Willd.) Iljin roots. Already since the late 80s, it has been delivered from Russia, first under the name Ecdysten (Ecdystenum) [72, 73, 81], now as highly purified 20-hydroxyecdysone forms named Ecdypure and Ecdybol.
In the late 90s, an advanced advertising campaign in western countries contributed to production of a large row of food additives on the ecdysterone basis (Russ Olimpic, Triboxin, Cytodyn ZM, Firm Ease etc.). As soon as the Rhaponticum carthamoides resources were exhausted, it was replaced by other 20-hydroxyecdysone containing plants (Achyranthes bidentata, Cyanotis arachnoidea, Pfaffia paniculata, Polypodium vulgare, Polypodium decumanum etc.). Since that the preparations made from chemically pure ecdys-teroids decreased in demand. Consumers started complaining of the instability of their effects. There appeared some critical papers, e.g. "The Truth about Ecdysteroids" considering synthetical ecdysteroids do not have any physiological effect and their activity is no more than a myth made by the advertising campaign.
Note. Today's world market is consistent of about 200 ecdysteroid-based commercial products, among them 149 are described in the works of Lafont and Dinan [39].
Actually, ecdysteroids were identified to compose not only higher flowering plants but also gymno-sperms, ferns, mushrooms, algae, mosses, as well as insects, crustaceans, and nematodes. According to the last investigations, almost all terrestrial and water higher plants have ecdysteroid-synthesizing genes [12, 66, 80].
Today we know the structure of more than 310 ecdysteroid molecules. Besides, angiosperms exceed in the diversity of kinds of ecdysteroids they contain. Insects have about 50 structure analogues of plant ecdys-teroids [61]. Among all various ecdysteroid molecules the mammals contain, the following three: ponasterone A, muristerone A, and ecdysterone are most active. Their structural formulas differ by quantity and arrangement of hydroxylic OH-groups, only. The first two ecdysteroids do not occur in the higher flowering plants. Ponasterone A is present in single ferns (including eagle fern - Pteridium aquilinum), mushrooms of the Paxillaceae family (Paxillus atrotomentosus), and relict plants of the Podocarpaceae and Taxaceae families (Fig. 6). Muristerone A is typical of the Ipomoea genus (morning-glory) from the Conovolvulaceae family (Fig. 7). Less active ecdysterone is wide-spread among flowering plants.
Dacridium

|
 Podocarpus elatus
Podocarpus elatus |
 Podocarpus nakaii
Podocarpus nakaii |
|
 Taxus baccata
Taxus baccata |
|
|
Fig. 6. The ponasterone A sources (Podocarpaceae and Taxaceae needles) | |
 |
 |
 |
 |
|
Fig. 7. The muristerone A sources (Ipomoea seeds) | |
 Polyporus umbellatus |
 Tapinella (Paxillus) panuoides |
|
Fig. 8. Mushrooms - sources of mycoecdysteroids | |
The biosynthesis pathways of plants, insects, and, possible, of mushrooms are different. Ecdysteroids are formed with acetate, mevalonate, cholesterol, ketol, ketodiol, ecdysone, ponasterone, 2,22-deoxyecdysone, 2,25-deoxyecdysone [49, 80]. At the initial stages of biosynthesis, a-ecdysone and ponasterone A are formed. Then, their molecules are oxidized to ecdysterone (b-ecdysone) that further can be trans-formed to other structures [70]. As a result of the fermentative modifications, we obtain cis- and trans-isomers of coupled A and B rings, epimers (compounds with a, b configurations of OH-groups). Plants possess ecdysteroids in form of water-soluble conjugates: conjugates with inorganic acids - sulphates, phos-phates; conjugates with organic (carbon, fatty, phenolic) acids - acetates, benzoates, cumarates; conjugates with sugars - glucosides, galactosides, xylosides; conjugates with acetone etc.
C27 ecdysteroids are characteristic of higher plants, C28 analogues are typical of mushrooms and gym-nosperms, while C29 compose gymnosperms. C30 ecdysteroids are very rare. As decay products of main ecdysteroids (C27...C29), there can be secondary C19...C24 structure analogues. The most wide-spread ecdysteroid is ecdysterone; polypodine B, inokosterone and ecdysone serve as additional major components in flowering plants; ecdysone, makisterone and ecdysterone in anthropoda; ponasterone A, pterosterone, and taxisterone in ferns and gymnosperms.
Apart from the above-mentioned main ecdysteroids, all the studied objects contain in trace amounts other structure analogues and their derivates (the so called minor ecdysteroids) numbering to 30-40 and more units. Some endemic and rare species, as well as those growing in specific ecological and geographical conditions, include ecdysteroids of unusual or abnormal structure, which do not occur in the most studied objects. The 90s are notable for isolation of ecdysteroids with new structures (polyporusterone A...G) from the Chinese bracket fungus (Polyporous umbellatus, Eichhase) in a quantity 0.1-3.0 mg/kg [29, 46]. It was also in this period when a new type of ergostane ecdysteroids (paxillosterone, atrotosterone, malakosterone) and their derivates from the mushrooms Tapinella panuoides and Paxillus atrotomentosus were obtained [64, 65]; (Fig. 8).
None of the mammal species contains ecdysteroids. The artificial chemical synthesis is only possible in the case of the secondary, biologically inactive or weakly active products via chemical transformation of major ecdysteroids. For this purpose, ecdysterone is mostly often used. Just recently the artificial photochemical transformation method was discovered allowing for structures being uncharacteristic of chemical transformation, e.g. dimers [23].
By the origin, it is accepted to divide ecdysteroid sources into phyto-, zoo-, and myco-ecdysteroids (i.e. plants; insects, cancroids, nematodes; mushrooms). Zooecdysteroids cannot be used for industrial production because of their utterly low concentrations in anthropoda. The value of this or that plant or mushroom species as a raw material source depends on its uniqueness grade formed by such indices as the biological activity, end use, concentration in biomass, availability, cost expediency [100].
|
||
|
Fig. 9. Agropopulation of Rhaponticum carthamoides (Willd.) Iljin (Photo Nikolay Timofeev; CF BIO) |

|
|
Fig. 10. Serratula coronata L. as an Ecdysterone (20-hydroxyecdysone, 20E) source |
The most important ecdysteroid sources for industrial isolation are plants, which, by their ability to biosynthesize ecdysteroids, can by classified as follows (on a dry weight basis):
In general, the differences among ecdysteroid concentration levels in plants are huge values - 8-9 orders (from 20-300 ng/kg to 20-30 g/kg). Normally, it is an extremely small value (thousandths and hundredths of a per cent on a dry weight basis). But there are plants which single organs in a restricted age and vegetation diapason can concentrate essential amounts of ecdysteroids. On average, among several thousands of other species there is one concentrator species. The species Rhaponticum carthamoides (Willd.) Iljin (Fig. 9) and Serratula coronata L. (Fig. 10) belong to the most important ecdysteroid-containing plants serving as industrial ecdysteroid sources. These species are considered to be highly promising in developing new classes of pharmaceutical preparations and biologically active food additives,and also ecologically safe products against pests [98, 103] ( http://www.ib.komisc.ru/t/ru/ir/vt/02-55/03.html).
Among angiosperms there is an insignificant number of other species with high content of ecdysteroids (mainly ecdysterone) in single organs, which are of interest for scientific purposes. Detailed investigations of the European northeast flora [66] showed the distribution of ecdysteroid-containing plants by taxons corresponding with the analogous distribution in other regions. The presence of these plants was identified in most species, whereas only 4% of them, represented by species with mean and high ecdysteroid content, developed a positive response in the radio-immune activity biotest. These data agree with the works done by other scientists where essential ecdysteroid concentrations belonged to 5-6 % of plants [61].
The species of secondary importance in the Russian flora are: some Silene and Lychnis varieties; Coronata flos-cuculi L.; Helleborus purpurascens and Helleborus caucasicus; Paris guadrifolia L.; Ajuga reptans; Sagina procumbens L.; Potamogeton natans and Potamogeton perfoliatus; Pulmonaria officinalis; Butomus umbellatus; Androsace filiforms etc [66].
Unfortunately, all these plants have some negative features, which do not make their industrial use possible. The main limiting factor is that they are difficult of access, grow in scattered groups or lonely, only wild and cannot be cultivated. Often they are low-height, creeping, rosulate, forest, meadow or water plants, poisonous or weakly toxic. They are met with in flood land thickets of meadow shrubs, forest edges and felled areas, peat-bogs, waste plots of land, along road and trench sides, at riverbanks and lake coasts or hill foots at high places. The life strategy of these plants is combined growing together with other species under the forest canopy, within meadows or as ruderal plants at cultivated fields. In most cases, introduction was not tried at all or represents serious difficulties.
Because isolation and purification of ecdysteroids from plant biomass is a complicated procedure heightening the prime cost of the end products, there have been developed ecdysteroid production technologies using biotechnological methods (cultures of cells, tissues, and transformed roots). Since ecdysteroid-biosynthesizing genes are present in all plant organs, callose cultures (cultures multiplying in the artificial nutritional medium) can be obtained from every quick-growing tissue: seed-lobs, hypocotyl, leaves, shoots, buds, roots. Ecdysterone and some other secondary important components of genera Ajuga, Serratula, Rhaponticum, Pteridium, Polypodium can be synthesized in cell culture. However, most active ecdysteroids, muristerone and ponasterone, cannot be synthesized in artificial conditions.
First studies on the Rhaponticum carthamoides callose and suspension cultures in Russia started about 10 years ago [83]. Ecdysteroid synthesis in callose cultures had an extremely low production rate. Also by other species, transition to plant cell cultivation was accompanied by a dramatical decrease of biosynthetical potential. On the whole, ecdysteroid content in cell culture is significantly lower than that in nature. A long-term cultivation lowers total content and changes the proportion ratio between individual compounds. Moreover, not identified ecdysteroids are synthesized. Ecdysterone concentrations in cell culture by different species comprise (on a dry weight basis): Rhaponticum carthamoides - 0.001-0.01; Serratula coronata - 0.02-0.09; Ajuga reptans - 0.015-0.1 [104]. The Japanese scientists were more successful with cell cultures from the Polypodium vulgare seedlings producing to 0.7 % ecdysterone [49].
The tissue culture technology on the Rhaponticum carthamoides seedlings implies placing aseptic explants to the nutritional medium, where a long-term cultivation allows production of actively multiplying homogenous parenchyma and meristem tissues, which do not regenerate into plant organs [87]. The callose tissue structure and growth rate depend on the nutrition and illumination factors combined. In general, ecdysteroid synthesis in suspension cultures has somewhat higher results than in cell culture, but these results are instable and ecdysterone concentration increases very slowly [80].
The most promising biotechnology method is a transformed roots culture. Inoculating sterile seedlings by the Agrobacterium rhizogenes strains causes an infection and agrobacterial transformation of roots to a hairy form. In case with Rhaponticum carthamoides the characteristic roots and swellings appear a month ago, they start spontaneous regeneration of modified plants. Within 4 weeks, hairy roots increase their mass by 4-6 times. Ecdysteroid fraction in culture, 0.02-0.03 % in total, differs from that of natural plant roots [84]. The hairy roots technology allows for ecdysterone biosynthesis of Ajuga reptans to 0.12 %, Serratula tinctoria 0.1-0.2 % [80].
The method of ecdysteroid production via hairy roots culture has its positive and negative features. Its advantage, compared to field culture, consists in a continuous reproduction source, high growth and regeneration rates of specimens; there is no need in external growth hormones as in the case with cell and tissue cultures. The nutrition source is saccharose. The method is applicable in respect to many cultures, modified roots are characterized by a high growth rate and genetic stability, large concentrations of secondary metabolites, comparable with that of natural plants. In addition to phytoecdysteroids, the secondary metabolites of hairy roots also involve alkaloids, polyacetilene compounds, glycosides, polyphenols, tannins, flavonoids, saponins etc.
However, the commercial use of such systems is restricted due to significant defects, which find a detailed treatment in the assay of Giri and Narasu [20]. The main restrictions arise from need for specially designed patterns of bioreactors with automatic control models to provide a free vertical culture development; these cultures need careful selection of optimal nutritional medium, temperature, and illumination conditions. One of the most important parameters, optimal roots morphology, influencing the density and aeration degrees of specimen is difficult to meet because many of various morphologies existing are in connection with different plasmid strains. Uniform aeration and intermixing procedures also provide certain difficulties, which lead to stagnant zones and fermentation, tissues necrosis and vital capacity loss. The end product accumulation is inhibited by its concentration saturation. It is necessary to filtrate and renew the liquid medium constantly. Also there are some difficulties appearing by crop collection and treatment, i.e. partially root removing from the reservoir etc.
Generally, ecdysteroid production by the biotechnology methods did not find large acceptance. Modified secondary ecdysteroids obtained by methods of biotechnology comprise less ecdysteroid activity indices, compared to nature. Therefore, such systems are used only to get chemically pure ecdysteroids. Besides, as the world experience readily shows, it is not enough just to have an ecdysteroid, it is to be highly active already in minimal concentrations, as muristerone A and ponasterone A are. Otherwise, chemically pure substances do not meet a ready sale. For example, only for several years the Chinese succeeded in putting ecdysterone isolated from Rhaponticum carthamoides genetically modified roots into mass production with the production volume exceeding that of Russia by thousands of times (Canfo Chemicals CO, Ltd; http://www.alibaba.com). At last, overproduction of ecdysterone resulted in a dramatic price decrease at the world market, and the technology became unprofitable.
![]()
![]() 5.1. Chemically pure ecdysteroids
5.1. Chemically pure ecdysteroids
The activity rate of isolated ecdysteroids is evaluated by biotesting with insect cells containing natural ecdysteroid receptors (EcR). Ponasterone A, muristerone A, and ecdysterone are regarded to be most active ecdysteroids with large practical use possibilities. Each of them can show different results with different receptors, but their initial activity rates are generally the same comprising 10-9 (10-8...10-10) M [22]. There are other ecdysteroids with 5, 6, 7 or even 8 OH-groups but relatively less active in isolated form. The following decreasing activity series was obtained: muristerone A, ponasterone A, polypodine B, 20E, 22-acetate 20E, 2-deohy-20E [67]. Concerning minor components some derivatives from muristerone (kaladasterone) and ajugasterone (dachryhainansterone) appeared active in biotests [5].
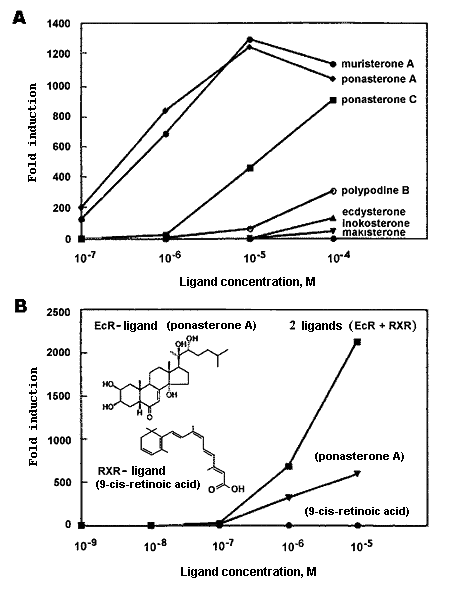
The activity rate of ecdysteroids in a real organism essentially differs from that in cell cultures, the doses are tissue-specific. Ecdysone-induced systems have effective doses of muristerone A and ponasterone A as single ligands in transgenic mice being equal to 10-5...10-7 M (Fig. 11A). Scientists give more preference to muristerone despite it is a rare and expensive substance ($ 120-135 for 1 mg). The ponasterone use is complicated because of its instability: after 3 hours the receptor complex decays by 50 % in buffer solution, whereas for muristerone the figures lie by 5 % [40].
 |
|
Fig. 11. The activity rates of isolated ecdysteroids (according to [51] changed): |
Concerning ecdysterone, though the biotests on insect cells revealed a significantly high bioactivity rate of 10-8 M [67], the ecdysone-induced systems are 2-3 orders of magnitude less in results. The activity rate of other ecdysteroids, polypodine B, ecdysterone, inokosterone, makisterone, is even more less, while a-ecdysone, 2-deoxyecdysone, 20-deoxyecdysterone, 22-acetate-ecdysterone do not have it at all [51].
Apart from EcR, none of the steroid receptors can interact with isolated ecdysteroids as ligands in mammal cells [15, 41]. This situation seems to be a satisfactory one because allows for avoiding negative side and unforeseen effects when using ecdysteroids as switch genes in the ecdysone-induced systems.
The anabolic activity of ecdysteroids, including the ability for protein synthesis inhibition or stimulation, was experimentally proven: a) in substances with insufficient purification degree (95 % and less); b) substances isolated from the producer plant Rhaponticum carthamoides; c) via unclear activation mechanisms with secondary agents [54, 91, 92, 101]. Numerous experiments in the fields of cell and molecular biology with individual highly purified compounds (99 %) and other ecdysteroid sources, e.g. Serratula coronata [85, 106], did not detected any signs of ecdysterone, muristerone, and ponasterone anabolic activity without secondary agents.
To activate gene transcription, hybrid ecdysteroid/retinoid receptors (EcR/RxR) and their modifications with other nuclear receptors are applied, where the RxR-partner is necessary to stabilize the heterodimer complex and to provide fixation of response elements before activation of gene expression mechanisms. In a living organism both ecdysteroid agonists and ligands of the heterodimer complex second partner (retinoid receptor) can act together, which enlarge their biological activity diapason significantly (Fig. 11B). Moreover, the effective doses decrease to 10-9...10-10 M under condition of a local treating a target organ [2].
The aforesaid is true only for highly purified (more than 98-99 %) formulations isolated from the primary sources. The artificial systems on their basis are extremely expensive and used mainly for scientific purposes. Mass ecdysteroid production in pharmaceutical industry can use non-purified or weakly purified plant formulations from non-toxic superproducer species, which do not require high-expensive ecdysteroid-possessing technologies.
Note Chemically pure ecdysteroids are obtained according to the following sequential procedures: raw material fining, extraction and extracts concentration by evaporation, watering, filtration, accompanying hydrophobic substances reextraction by hydrocarbon solvent, ballast compounds precipitation, phytoecdysteroids extraction from water portion with evapoconcentration, chromatographic purification in column with aluminum oxide, eluate evapoconcentration, selective elution of ecdysteroids from sorbent material with solvent and resulted fractions concentration, re-crystallization and vacuum drying after dissolution in metanole and evapoconcentration, or filtration after freezing at -40 to -70°C [13, 17, 19, 52, 75, 81, 86, 90, 105].
![]()
![]() 5.2. Non-purified Rhaponticum carthamoides formulations
5.2. Non-purified Rhaponticum carthamoides formulations
Chemically isolated ecdysteroid fraction (91 %, including 75 % 20-hydroxyecdysone) extracted from the overground Serratula coronata shoots demonstrated a complex and ambiguous "dose-effect" modulation activity (also called two-phase operation) in the spontaneous E-rosette-forming biotest within the concentration limits 10-4...10-12 M [102]. The effective immuno-modulating activity rate of CD2+-rosette forming with human T-lymphocytes was achieved by a concentration value of 1µ M (10-6 M) and a stimulation index of 1.132 [58].
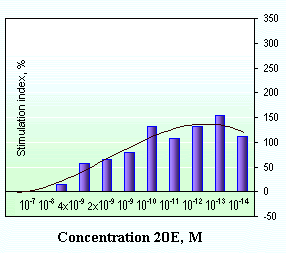
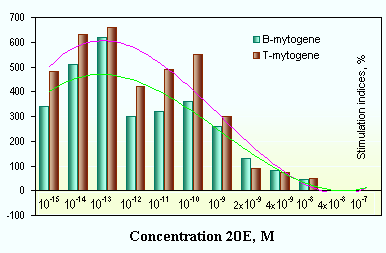
Natural ecdysteroid-containing substances can be significantly more active than chemically isolated ecdysteroids (Fig. 12). Cultivating lymphocyte populations in vitro in presence of the Rhaponticum carthamoides extract can cause proliferation of spleen cells in concentration 10-13...10-14 M. The nonspecifically activating ConA (T-mytogene) and LPS (B-mytogene) agents stimulate proliferation up to 10-15 M [107].
|
 |
|
Fig. 12. Cell proliferation stimulation by Rhaponticum carthamoides extract: | |
At present, a new class of ecdysteroid-containing pharmpreparations with super-low doses of active substances is being worked out (http://www.kstu.ru/jchem&cs/russian/n5/1vr29/29.htm). The new preparations are produced from the overground Rhaponticum carthamoides shoots grown according to a special technology in agropopulations (http://homepages.atnet.ru/timfbio/randser.htm) [95, 96, 99]. The crude drug used in production allows decreasing today doses - by 3-4 orders of magnitude, if 20-hydroxyecdysone [97].
For example, effective doses of the "Bioinfusin" and "BCL-PHYTO" (BCL - BactoCelloLactin) pharmpreparations account for 0.5-10.0 microgram/Kg biomass (10-12...2x10-13 M), for ecdysterone [76, 98]. This is not a mistake or misprint because an average daily dose of chemically pure 20-hydroxyecdysone and preparations on its basis is 5-50 milligram/Kg body weight [35, 43, 69, 74, 79, 85, 91, 101, 106].
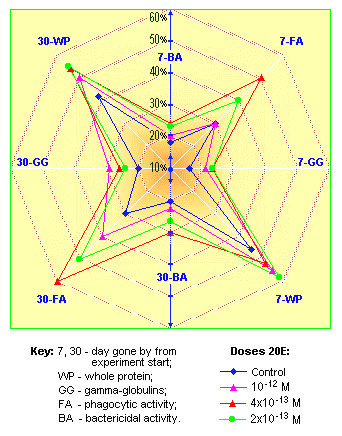
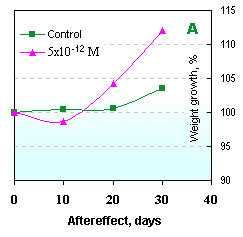
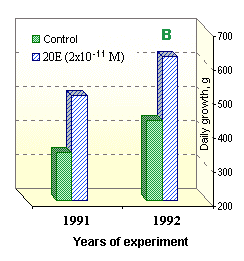
The action mechanism specificity of new preparations lies in a stimulating activity of small doses and inhibiting effects on proliferative organism processes in large doses. Even one-time introduction of such drugs can cause essential immuno-stimulating effects on cell and humoral levels [76, 77, 94]. The seven-day-long course of treatment allows for considerable immuno-stimulating aftereffects, which last for 30 days (Fig. 13). Besides, non-purified ecdysteroid formulations possess a stable industrial anabolic effect when used for mass industrial production (Fig. 14).
Note:
 |
|
Fig. 13. Immuno-modulating effect of the "Bioinfusin" preparation |
 |
 |
|
Fig. 14. Anabolic effect of small ecdysteroid doses: | |
Research on ecdysteroids is a direction in biology offering rich possibilities for fundamental and practical scientific studies. Exploring the role and mechanisms of ecdysteroid biological activity allows for a real possibility to realize the boldest human projects, i.e. learn how to get control over the vital activity of different organisms manipulating the activity rate of the particular genes according to the "switch on-switch off" principle. Practically, it would help to get rid of whole number incurable diseases and refuse the chemical synthesis for the ecologically safe biological synthesis of many important substances.
Research on ecdysteroids is widely represented by Internet, including data on the genetics; cell and molecular biology; human, animal, and plant physiology, as well as commercial offers intended for solving real questions in chemistry, biotechnology, pharmacology, medicine, entology, and agriculture. Ecdysteroids are investigated by the biggest world laboratories. Different states offer as their sources native plant species: ferns, convolvuluses, conifers, yew-trees, and amaranths.
In the case of Russia, cultivating Rhaponticum and Serratula plants representing super concentrator plant species is economically sound. The basic ecdysterone concentrations in plants of Rhaponticum carthamoides (Wlld.) Iljin and Serratula coronata L. comprise 0.12-0.57 % and 0.31-1.15 % dry weight, respectively. Regarding the former species, there appeared an industrial growing technology and a new class of pharmaceutical preparations from its aboveground shoots is being developed. The latter is under introduction study in different Russian regions.
To satisfy the mass demand for ecdysteroids in the pharmaceutical industry, non-purified or weakly purified plant formulations from super producer species with a null toxicity rate that do not require high-expensive processing technologies have good prospects. The effective biological activity rate of extracts from Rhaponticum carthamoides grown with a special technology in agropopulations accounts for for 10-11...10-13 M. It is about 3-4 orders of magnitude higher than the activity rate of highly purified individual ecdysteroids. Stable results of comparable doses were experimentally obtained in biotests, tests on laboratory animals, and in conditions of a large-scale production. What is particularly responsible for the unusually high activity rate of Rhaponticum carthamoides has to be looked for in its complex chemical composition causing the complex biological activity of ecdysteroids with other metabolites.
ACKNOLEGMENTS
The author thanks Elena S. Kuzmina (Institute of Biology Komi SC UrD RAS, Syktyvkar, Russia) for translation of the manuscript.